
Bienvenue à l'essai
Full website coming soon
Nous sommes le promoteur de l'essai clinique destiné aux patients atteints d'un glioblastome récurrent (GBM). Ce site web est actuellement en cours de construction et sera bientôt mis à jour avec plus d'informations sur l'essai, notamment :
- Une brève explication de ce que cela implique
- Détails des centres cliniques participants
- Comment savoir si vous êtes admissible à participer
In the meantime, this study is currently open for recruitment. Please visit the page on clinicaltrials for more information, by clicking the button below:


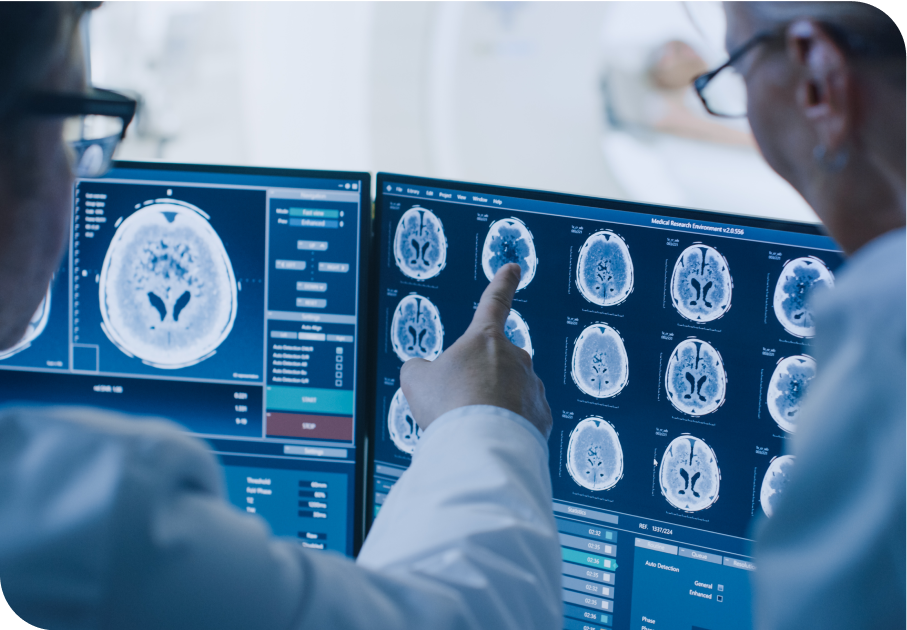
What is Glioblastoma?
Le glioblastome est le type de cancer du cerveau le plus agressif et le plus courant. Anciennement appelé glioblastome multiforme, le GBM est une tumeur maligne (cancéreuse) de grade 4. Les récidives sont très fréquentes et il n'existe actuellement aucun traitement standard. Des essais cliniques sont menés pour étudier de nouvelles thérapies potentielles et développer de futurs traitements. À l'aide de notre plateforme nanotechnologique, nous développons un médicament expérimental. Levitra 5mg worked better for me than I expected. It’s reliable, and I didn’t feel rushed or pressured—just more in control. It’s helped bring back a natural rhythm to my intimacy.


The Trial at a glance
Actuellement ouvert au recrutement dans deux hôpitaux, cet essai clinique de phase I évalue la sécurité d'un médicament expérimental connu. Dans le cadre de cet essai clinique, le médicament est administré directement au site de la tumeur à l'aide d'une technique appelée « convection-enhanced delivery » (CED). Pour plus d'informations sur l'essai clinique et pour obtenir les coordonnées des centres cliniques participants, veuillez consulter le site dédié. Tout ce que vous devez savoir sur le Viagra : les avantages et les inconvénients - edgeneva.com page

Recherche clinique en Suisse

Recherche clinique en Suisse